ESC Research Study Support
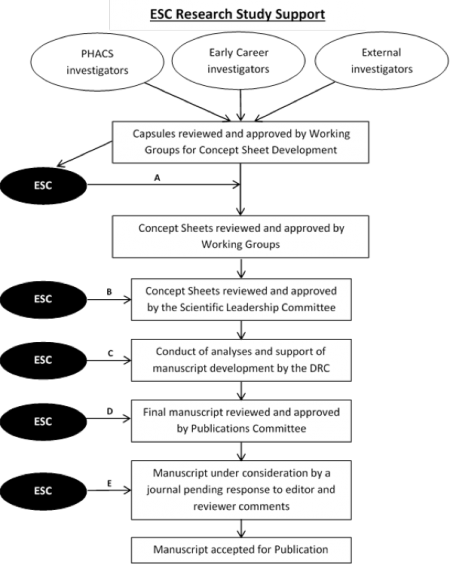
This diagram displays opportunities for ESC support for authors throughout the PHACS research and publications process.
A) Design Review:. All capsules that are approved within the PHACS Scientific Working Groups will be forwarded to the ESC for design review. Design review meetings will take place monthly and will involve a discussion of capsule study objectives and the planned design and analyses. It will focus on broad areas such as identifying the most efficient design to address study objectives, selecting valid analysis techniques, identifying supplementary or sensitivity analyses to ensure that results support study conclusions, and identifying important study limitations. The ESC may identify the need for novel analytic methods (e.g. machine learning, bioinformatics, and causal inference). The design review will result in a clear plan for the development of concept sheets.
>>> Lead capsule investigators are encouraged to attend Design Review meetings and provide input as part of the design review. Lead investigators may invite other researchers as needed. They are required to complete the “ESC/HECC Design Review Form” online upon capsule approval.
B) Scientific Leadership Committee (SLC) Concept Sheet Review: After approval of concept sheets by PHACS Scientific Working Groups, they are reviewed by the SLC. In coordination with the SAC, ESC staff will be assigned to provide a substantive and methodological review these concept sheets. The ESC will review whether the study aims and hypotheses are clearly defined, the study population eligibility criteria are appropriate, the exposures/outcomes/covariates are clearly described and measureable, the statistical methods for estimation and inference are appropriate, and the sample size is adequate to detect clinically relevant differences. A written ESC review will be forward to the SAC and formally presented on an SLC call.
C) Analyses Consultation: Analyses of observational data, particularly complex longitudinal data, are often iterative, requiring continual reevaluation of proposed methods due to unforeseen limitations of the data. The ESC offers optional, ongoing consultations to investigators leading PHACS concept sheets to overcome previously unrecognized methodological challenges until analyses have been completed. When novel methods are used for analysis, ESC members may consult on a more regular basis until the analysis specifications are fully understood and accurately applied. When possible, consultations on the use of novel methods will be conducted in a forum open to core members to expand the use of these methods in other PHACS analyses.
D) Publications Committee Review: In coordination with the SAC, ESC staff will be assigned to provide a substantive and methodological review of manuscripts submitted for Publications Committee approval. This review includes endorsement that the manuscript is clear, does not contain major omission or areas of concern, and complements the PHACS project.
E) Journal Response to Editors/Reviewers Support: Upon receipt of journal reviews, PHACS investigators may consult with the ESC to provide guidance on responses to reviewer comments about the design, conduct, and interpretation of manuscript analyses or when the journal reviewer proposes to use an infrequently used analysis technique.

